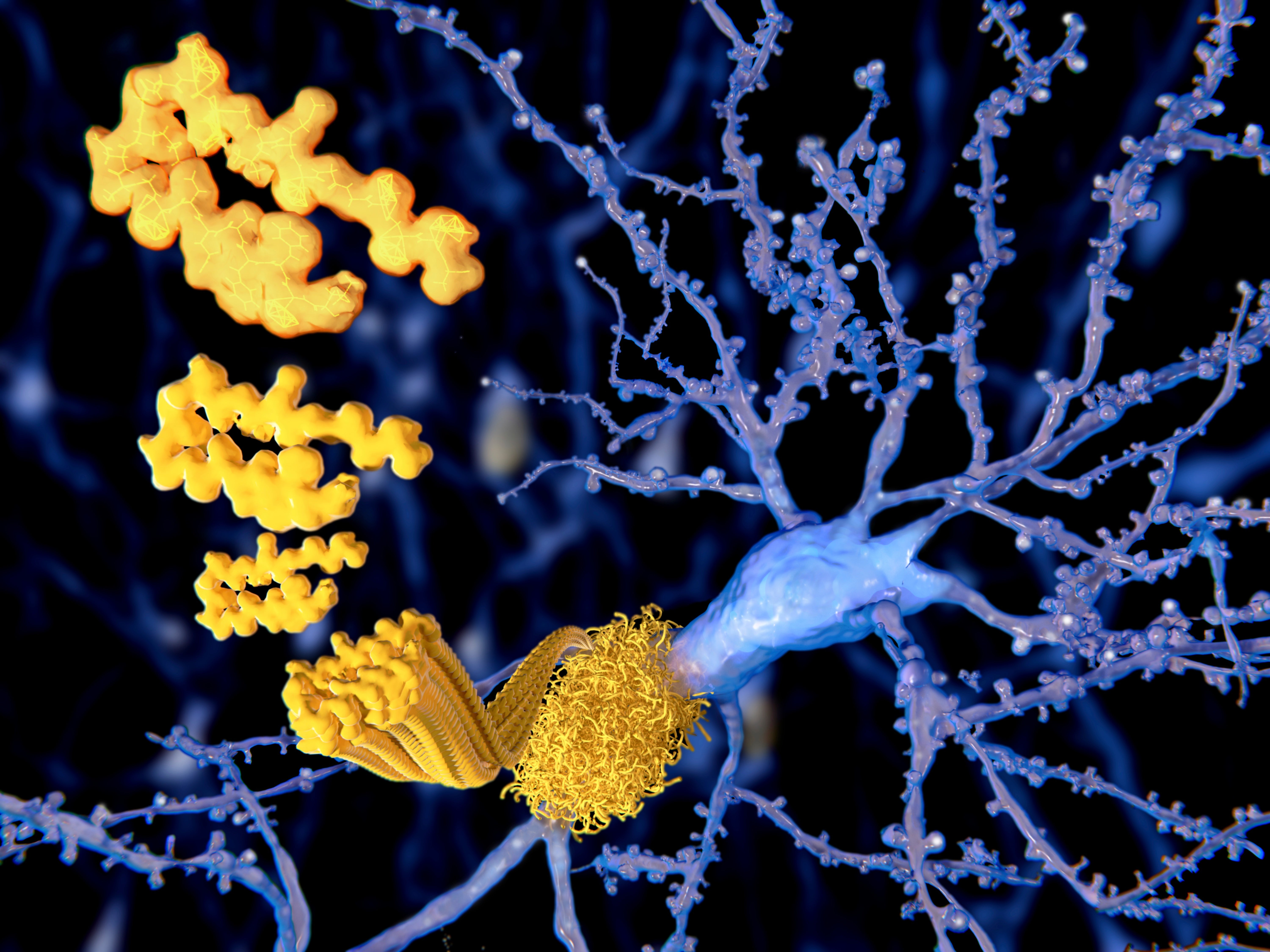
Alzheimer’s Drug Candidate Receives Favorable FDA Panel Review
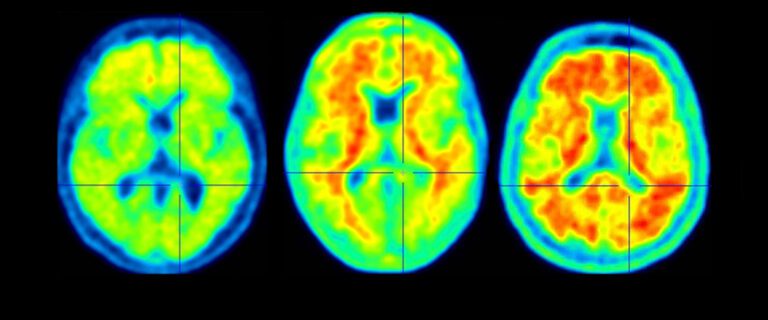
A groundbreaking drug candidate that aims to slow the progression of Alzheimer’s disease has received a positive recommendation from an advisory panel to the U.S. Food and Drug Administration (FDA). The drug, lecanemab, is a monoclonal antibody that targets amyloid-beta plaques, harmful protein deposits that accumulate in the brains of Alzheimer’s patients. In clinical trials, lecanemab has shown promise in reducing amyloid-beta levels and potentially slowing the cognitive decline associated with the disease. The FDA’s Peripheral and Central Nervous System Drugs Advisory Committee voted 8-1 in favor of recommending approval of lecanemab for the treatment of Alzheimer’s. The committee acknowledged the drug’s potential benefits, including its ability to reduce amyloid-beta plaques and potentially slow disease progression. “This is a significant moment in the fight against Alzheimer’s disease,” said Dr. Peter Passmore, director of the Alzheimer’s Association of Greater Austin. “Lecanemab has the potential to be the first drug approved to treat the underlying cause of Alzheimer’s, not just its symptoms.” However, the panel also raised concerns about the drug’s safety, particularly the risk of side effects such as brain swelling and bleeding. They recommended that the FDA require more data on safety before approving the drug for general use. “The panel recognized the potential of lecanemab, but they also emphasized the need for continued evaluation of its safety,” said FDA Commissioner Robert M. Califf, M.D. “We will carefully consider the committee’s recommendations as we make our final decision on whether to approve lecanemab.” The FDA is expected to make a final decision on lecanemab’s approval by early January 2023. If approved, it would mark a major milestone in the fight against Alzheimer’s disease, which affects over 6 million Americans.Alzheimer’s Drug That Could Slow the Disease Gets Support From FDA Advisers A panel of experts convened by the U.S. Food and Drug Administration voted overwhelmingly to recommend that the agency approve a new drug for Alzheimer’s disease. The drug, called aducanumab, is the first to show promise in slowing the progression of the disease. In a 10-0 vote, the panel recommended that the FDA approve aducanumab for people with mild cognitive impairment or mild dementia due to Alzheimer’s disease. The FDA is not required to follow the advice of its advisory panels, but it usually does. Aducanumab is a monoclonal antibody that targets amyloid beta, a protein that forms plaques in the brains of people with Alzheimer’s disease. These plaques are thought to contribute to the memory loss and cognitive decline that are characteristic of the disease. In a clinical trial, aducanumab was shown to reduce amyloid beta plaques in the brains of people with Alzheimer’s disease. The trial also found that aducanumab slowed the progression of cognitive decline in these patients. The FDA is expected to make a final decision on whether to approve aducanumab by March 7, 2021. If approved, aducanumab would be the first new Alzheimer’s drug to be approved in nearly two decades. The news of the FDA advisory panel’s recommendation is a major development in the fight against Alzheimer’s disease. Aducanumab is the first drug to show promise in slowing the progression of the disease, and its approval would be a significant step forward in the search for a cure.