A recent study published in the journal Nutrients provides a comprehensive overview of the lipoprotein pathway in healthy metabolism.
 Study: Effects of lipoproteins on metabolic health. Image credit: Nemes Laszlo / Shutterstock.com
Study: Effects of lipoproteins on metabolic health. Image credit: Nemes Laszlo / Shutterstock.com
What are lipoproteins?
Lipoproteins consist of lipids chemically bound to protein conjugates called apolipoproteins. These amphipathic molecules have a central lipid core of cholesterol esters and triacylglycerol within a double membrane of phospholipids and free cholesterol conjugated to apolipoproteins.
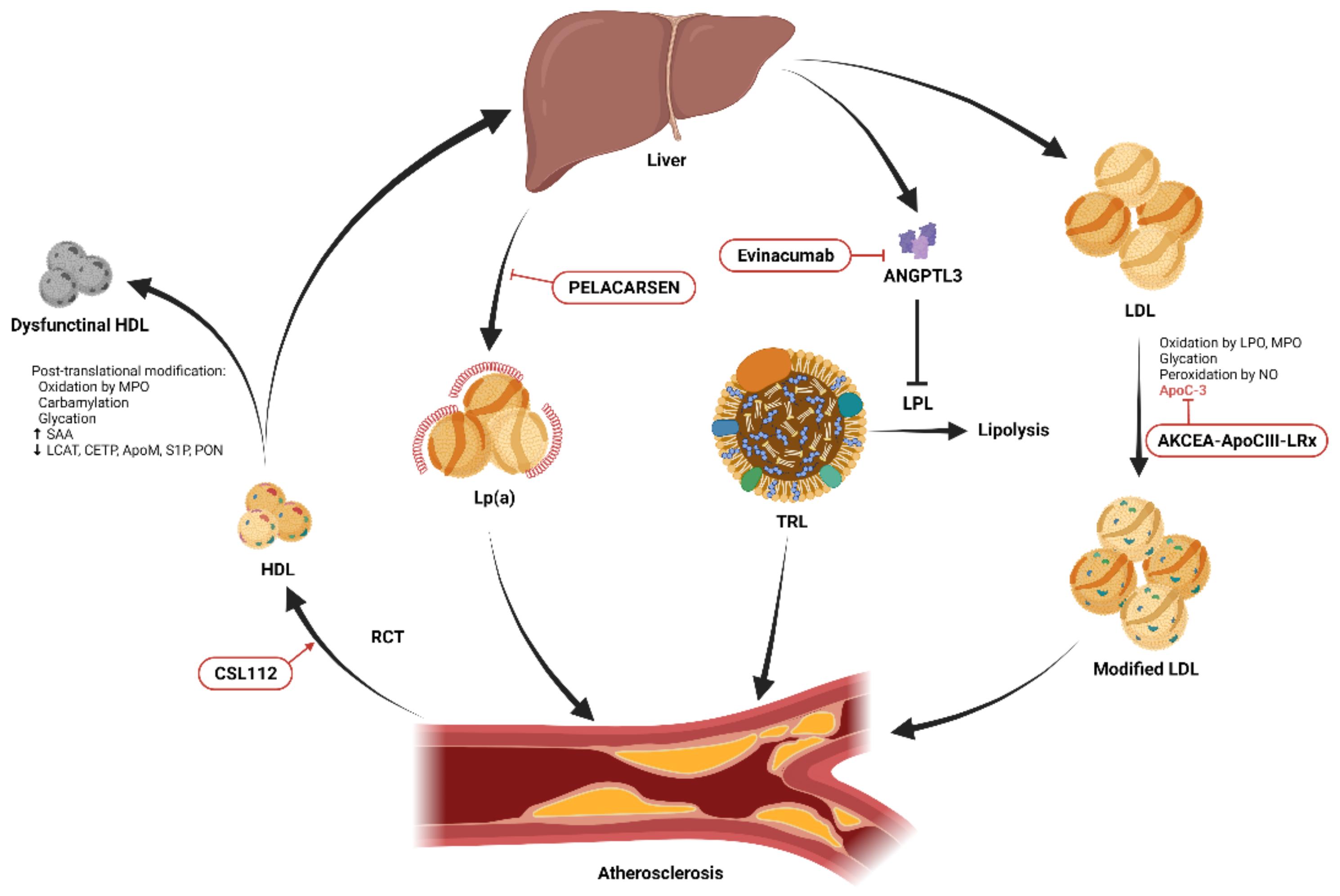
Some examples of lipoproteins are high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), and lipoprotein(a) (LP(a)), with the density of these molecules being inversely proportional to their size. HDL and LDL are the smallest lipoproteins at eight to twelve nanometers (nm) and 18-25 nm, respectively, while chylomicrons are the largest at 100-1200 nm.
Dietary lipids are digested in the gastrointestinal tract, where they enter the intestinal mucosa to become chylomicrons, which transport triacylglycerols from food into the blood. Chylomicron breakdown in muscle or fat tissue yields chylomicron remnants for excretion by liver cells.
Lipoprotein synthesis occurs in the liver and begins with the production of VLDL, which contains triglycerides. After further processing, these triglycerides become intermediate-density lipoproteins (IDL) and then LDL or LP(a).
VLDL transports triacylglycerols produced by the body, while LDL transports cholesterol from the liver to other tissues. LDL initiates the formation of atherosclerotic plaque and is therefore a biomarker for cardiovascular disease (CVD) risk. HDL transports cholesterol from peripheral tissues to the liver via reverse cholesterol transport (RCT).
Lipoprotein synthesis is followed by its secretion into the bloodstream, transport, modification and elimination from the body. Liver cells and macrophages obtain lipoproteins by endocytosis to break down or use these molecules.
Lipoproteins and CVD
The primary route of blood LDL management is via macrophage uptake of cholesterol esters. Within the macrophage, cholesterol esters are eventually converted to foam cells, which serve as free cholesterol for storage or exit the cell via transporters.
In addition to high cholesterol and reduced macrophage foam cell migration, high LDL worsens atherosclerosis, which is exacerbated by genetic and metabolic factors. Increased expression of oxidized LDL 1 (LOX-1) can lead to plaque instability, thrombosis, and acute CVD.
Atherosclerosis also causes inflammation through the activation of pattern recognition receptors by oxidative stress. These effects are particularly evident in obese individuals, but also in elderly individuals and diabetics.
Oxidized LDL can lead to leukocyte adhesion, activate apoptosis and cause endothelial dysfunction. In addition, it increases collagen synthesis and cell proliferation by fibroblasts and vascular smooth muscle cells.
Atherogenic dyslipidemia is common in obesity and increases the risk of diabetes mellitus and CVD. Although HDL levels are low in both obesity and diabetic dyslipidemia, triglyceride-rich lipoproteins such as LDL are high.
Treatment of dyslipidemia
Dyslipidemia increases the risk of CVD, especially in the presence of type 2 diabetes, as well as tumor development and neurodegenerative diseases such as Alzheimer’s dementia. Dyslipidemia can also lead to high cholesterol, obesity, atherosclerosis, inflammation and insulin resistance.
Importantly, both genetic and dietary factors influence the incidence of dyslipidemia. For example, chylomicron storage disease and abetalipoproteinemia can disrupt lipid absorption.
Although many risk factors for dyslipoproteinemia are immutable, such as aging, gender, ethnicity, or family history of diseases caused by genetic mutations, several novel therapies are being investigated. For example, gene therapy offers a promising approach to modifying the pathogenic genes involved in the development of dyslipidemia. Recent animal studies have shown promising results using gene editing technologies to reduce circulating LDL and protein convertase subtilisin/kexin type 9 (PCSK9) levels.
PCSK9 is an enzyme that promotes clotting, leukocyte recruitment, and platelet activation in the bloodstream, thereby promoting CVD progression and affecting the availability of LDL receptors. PCSK9 antibodies, including alirocumab, evolocumab, and the ribonucleic acid (RNA) molecule inclisiran, have been shown to lower LDL levels.
Statins inhibit cholesterol synthesis and thus lower LDL levels, while ezetimibe and fibrates inhibit lipid absorption.
Novel antidiabetic therapies, including dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide 1Ra (GLP-1Ra) agonists, and GLP-1 and glucose-dependent insulinotropic peptide (GIP) dual receptor agonists, have also been shown to be effective in treating dyslipidemia. These medications can be used with or without lipid-lowering agents, which may support optimization of treatment regimens, improve patient adherence, and lead to better outcomes.
The relationship between lipid metabolism and microbes in the intestinal lumen is an emerging area of research that may also lead to the development of novel therapies.
RCT is a potential treatment method for CVD; however, no data are available to support the correlation between HDL and CVD. Apolipoprotein E (apoE) is also essential for RCT, as it contributes to the removal of LDL together with apoB. ApoE analogues provide another route to improve RCT and prevent atherogenesis.
Journal reference:
- Albitar, O., D’Souza, & Adeghate, E. A. (2024). Effects of lipoproteins on metabolic health. Nutrients. doi:10.3390/nu16132156.