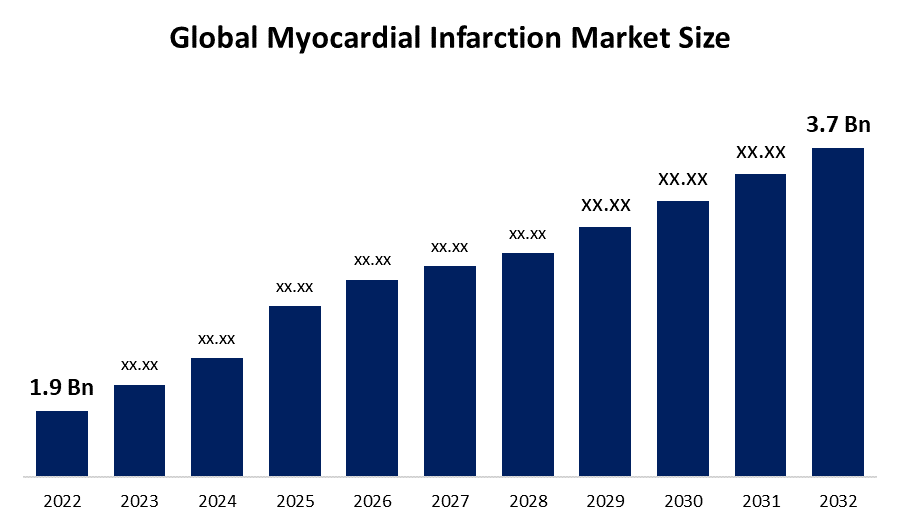
The myocardial infarction market size reached a value of USD 1.4 Billion in 2023. Looking forward, the market is expected to reach USD 2.4 Billion by 2034, exhibiting a growth rate (CAGR) of 4.94% during 2024-2034.
The market is driven by the introduction of innovative therapies along with diagnostic technologies. Additionally, novel anticoagulants as well as antiplatelet drugs are improving post-infarction management by reducing the risk of subsequent events.
Advancements in Stent Technologies: Driving the Myocardial Infarction Market
One of the major trends revolutionizing the myocardial infarction market is advancements in stent technologies which are predominantly impacting the treatment as well as management of myocardial infarction, providing better outcomes for patients with coronary artery disease. In line with this, the development of next-generation drug-eluting stents (DES). These stents release medication that prevents the re-narrowing of arteries, addressing a major limitation of earlier stent models. For instance, the Xience family of stents by Abbott has shown remarkable efficacy and safety in reducing restenosis rates and improving long-term clinical outcomes. These stents use everolimus, an anti-proliferative drug, to inhibit the growth of scar tissue within the artery, thereby maintaining vessel patency. Another groundbreaking advancement is the introduction of bioresorbable vascular scaffolds (BVS). Unlike traditional metal stents, BVS is designed to dissolve gradually after restoring blood flow, leaving behind a natural vessel. This innovation aims to reduce long-term complications associated with permanent implants, such as chronic inflammation and late stent thrombosis. The Absorb GT1 BVS by Abbott, though facing initial challenges, has spurred further research and development in the field of bioresorbable technologies. Clinical studies have demonstrated that when used in appropriately selected patients, these scaffolds can significantly improve long-term outcomes and vessel healing.

Request a PDF Sample Report: https://www.imarcgroup.com/myocardial-infarction-market/requestsample
Additionally, hybrid stents that combine the benefits of drug-elution and bioresorbable materials are being explored. These stents aim to provide immediate support and drug delivery, followed by gradual resorption, which helps restore natural vessel function over time. For instance, the MAGMARIS magnesium scaffold by Biotronik represents a significant step forward, offering both mechanical strength and bioresorbable properties, which are pivotal for long-term success in myocardial infarction management. These advancements in stent technologies are crucial for improving the prognosis of myocardial infarction patients. By reducing complications and enhancing the natural healing process of arteries, these innovations are transforming the landscape of interventional cardiology, providing more effective and safer treatment options for patients worldwide.
Novel Pharmacological Therapies: Contributing to Market Expansion
Advancements in novel pharmacological therapies are significantly improving the management and outcomes of myocardial infarction (MI), offering new hope for patients through enhanced efficacy and safety profiles. One major development is the introduction of next-generation antiplatelet agents, which play a critical role in preventing thrombotic events post-MI. For example, ticagrelor (Brilinta) is an antiplatelet drug that is more effective than clopidogrel in reducing the risk of cardiovascular death, MI, and stroke in patients with acute coronary syndromes (ACS). The PLATO trial demonstrated that ticagrelor significantly reduced the rate of these events compared to clopidogrel, highlighting its superior efficacy in high-risk patients. Another significant advancement is the development of novel anticoagulants that offer better management of thrombosis with fewer bleeding complications. Rivaroxaban (Xarelto), an oral Factor Xa inhibitor, has been incorporated into treatment regimens for patients with MI to prevent further ischemic events. The ATLAS ACS 2-TIMI 51 trial showed that low-dose rivaroxaban, when added to standard antiplatelet therapy, reduced the risk of death from cardiovascular causes, MI, and stroke in patients with recent ACS. This combination therapy approach provides a dual mechanism of action, targeting both platelet aggregation and thrombin generation, thus offering comprehensive protection against recurrent events.
Additionally, PCSK9 inhibitors, such as alirocumab (Praluent) and evolocumab (Repatha), represent a novel class of lipid-lowering agents that significantly reduce low-density lipoprotein cholesterol (LDL-C) levels. These drugs have demonstrated efficacy in lowering the incidence of major cardiovascular events in patients with a history of MI. For instance, the FOURIER trial revealed that evolocumab, in conjunction with statin therapy, significantly lowered the risk of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. These novel pharmacological therapies are transforming the myocardial infarction market by providing more effective and safer treatment options. Their ability to reduce the risk of recurrent events and improve long-term outcomes is making a profound impact on patient care, contributing to better survival rates and quality of life for those affected by myocardial infarction.
Integration of Digital Health Tools:
The integration of digital health tools in the myocardial infarction market is revolutionizing patient care, and enhancing monitoring, treatment adherence, and overall outcomes. One prominent example is the use of wearable devices that continuously monitor vital signs, such as heart rate and rhythm, which are critical for patients recovering from a myocardial infarction (MI). Devices like the Apple Watch and Fitbit not only track physical activity but also can detect irregular heart rhythms and alert users to seek medical attention promptly. These tools enable early detection of potential complications, allowing for timely intervention and reducing the risk of subsequent cardiac events. Telemedicine platforms have also become integral in managing MI patients, particularly in the follow-up phase. Platforms such as Teladoc and Amwell provide remote consultations, allowing patients to receive care from cardiologists without the need for in-person visits. This is especially beneficial for the continuous management of medication regimens and lifestyle adjustments necessary for recovery. For instance, studies have shown that telemedicine can improve medication adherence and lifestyle modifications in MI patients, leading to better clinical outcomes. Additionally, mobile health apps like MyHeart and Cardiogram offer educational resources, medication reminders, and symptom tracking, which empower patients to actively manage their condition.
Furthermore, digital platforms are being used to enhance cardiac rehabilitation programs. Traditional rehabilitation requires frequent visits to specialized centers, which can be a barrier for many patients. Digital cardiac rehabilitation programs, like those offered by apps such as Kaia Health and Moving Analytics, provide structured exercise routines, dietary recommendations, and stress management techniques that patients can follow from home. These programs are tailored to individual patient needs and have been shown to improve participation rates and cardiovascular health outcomes. The integration of these digital health tools reflects a broader trend towards personalized and accessible healthcare, improving the management of myocardial infarction. By leveraging technology, healthcare providers can offer more efficient, continuous, and patient-centered care, ultimately enhancing the quality of life for MI patients.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7819&method=587
Leading Companies in the Myocardial Infarction Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global myocardial infarction market, several leading pharmaceutical and medical device companies are at the forefront, each contributing innovative treatments and technologies to improve patient outcomes. Some of the major players include Sanofi, Chiesi USA, Inc, and Amgen. These companies have been investing heavily in their manufacturing capacities in recent months.
Sanofi has recently announced significant advancements related to Lovenox (enoxaparin sodium), particularly in its applications for severe myocardial infarction (MI). The FDA has approved Lovenox for use in treating the most severe types of heart attacks, highlighting its efficacy and safety in critical cardiovascular conditions. This approval underscores the drug’s importance in reducing the risk of venous thromboembolism (VTE) and other thrombotic events in high-risk patients, such as those suffering from acute ischemic stroke.
Moreover, Chiesi USA is another major player who has announced the successful acquisition of full commercial rights to Retavase in the United States from Roche. This acquisition allows Chiesi to expand its portfolio and strengthen its presence in the acute cardiovascular care market. The company plans to leverage its expertise and resources to maximize the impact of Retavase in treating acute myocardial infarction.
Apart from this, Amgen has recently highlighted significant findings related to its PCSK9 inhibitor, Repatha (evolocumab), which are poised to impact the myocardial infarction market substantially. Data from the Phase 3 FOURIER Open Label Extension (FOURIER-OLE) studies have shown that long-term use of Repatha continues to provide substantial reductions in low-density lipoprotein cholesterol (LDL-C) levels without new safety concerns. Over five years, more than 85% of patients achieved LDL-C levels below 40 mg/dL, demonstrating the drug’s sustained efficacy in managing cholesterol and reducing cardiovascular risks, including heart attacks and strokes.
Request for customization: https://www.imarcgroup.com/request?type=report&id=7819&flag=E
Regional Analysis:
The major markets for myocardial infarction include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for myocardial infarction while also representing the biggest market for its treatment. This can be attributed to advancements in treatment options, increasing prevalence of cardiovascular diseases, and heightened awareness of heart health.
Moreover, a high prevalence of cardiovascular risk factors includes hypertension, hyperlipidemia, diabetes, and obesity. According to the Centers for Disease Control and Prevention (CDC), approximately 805,000 Americans suffer a heart attack each year, with one in five being silent, where the damage is done but the person is not aware of it.
Besides this, advancements in stent technologies are also crucial in the myocardial infarction market. The development of drug-eluting stents (DES) and bioresorbable vascular scaffolds (BVS) has greatly improved long-term outcomes for patients undergoing percutaneous coronary intervention (PCI). Abbott’s Xience stents, for example, have been instrumental in reducing restenosis rates and improving patient recovery.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the myocardial infarction market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the myocardial infarction market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current myocardial infarction marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/myocardial-infarction-market
IMARC Group Offer Other Reports:
Medical Videoscopes Market: The global medical videoscopes market size reached US$ 26.1 Billion in 2023, and projected to reach US$ 43.35 Billion by 2032, exhibiting a growth rate (CAGR) of 5.6% during the forecast period from 2024 to 2032.
Vaccine Contract Manufacturing Market: The global vaccine contract manufacturing market size reached US$ 2.8 Billion in 2023, and projected to reach US$ 6.1 Billion by 2032, exhibiting a growth rate (CAGR) of 8.8% during the forecast period from 2024 to 2032.
Orthopedic Software Market: The global orthopedic software market size reached US$ 330.4 Million in 2023, and projected to reach US$ 522.3 Million by 2032, exhibiting a growth rate (CAGR) of 5.1% during the forecast period from 2024 to 2032.
Diabetic Retinopathy Market: The global diabetic retinopathy market size reached US$ 8.1 Billion in 2023, and projected to reach US$ 13.0 Billion by 2032, exhibiting a growth rate (CAGR) of 5.2% during the forecast period from 2024 to 2032.
Respiratory Syncytial Virus (RSV) Diagnostics Market: The global respiratory syncytial virus (RSV) diagnostics market size reached US$ 1,005.2 Million in 2023, and projected to reach US$ 2,072.3 Million by 2032 exhibiting a growth rate (CAGR) of 8.1% during the forecast period from 2024 to 2032.
Viral Inactivation Market: The global viral inactivation market size reached US$ 635.6 Million in 2023, and projected to reach US$ 1,390.8 Million by 2032, exhibiting a growth rate (CAGR) of 8.8% during the forecast period from 2024 to 2032.
Endoscopy Devices Market:
The global endoscopy devices market size reached US$ 47.1 Billion in 2023, and projected to reach US$ 82.2 Billion by 2032, exhibiting a growth rate (CAGR) of 6.2% during the forecast period from 2024 to 2032.
Transdermal Drug Delivery Systems Market: The global transdermal drug delivery systems market size reached US$ 6.7 Billion in 2023, and projected to reach US$ 11.1 Billion by 2032, exhibiting a growth rate (CAGR) of 5.5% during the forecast period from 2024 to 2032.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
Phone Number: – +1 631 791 1145, +91-120-433-0800