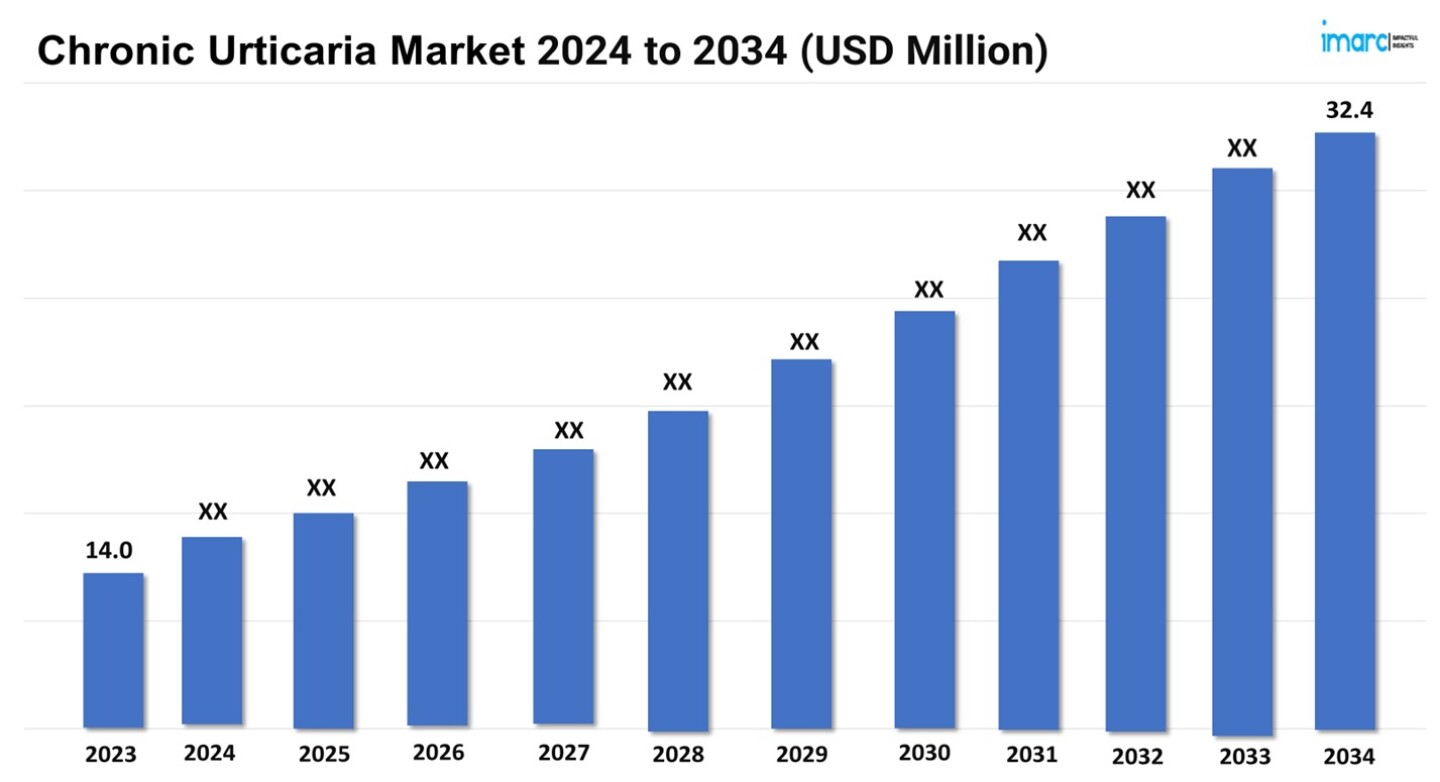
The chronic urticaria market size reached a value of USD 14.0 Million in 2023. Looking forward, the market is expected reach USD 32.4 Million by 2034, exhibiting a growth rate (CAGR) of 7.97% during 2024-2034.
The market is driven by increasing prevalence, advancements in treatment options, and a greater focus on patient-centric care. Additionally, the adoption of biologic therapies, which offer targeted treatment and long-lasting relief, and the integration of digital health solutions to enhance patient management further propel the market expansion.
Advancements in Biologic Therapies: Driving the Chronic Urticaria Market
Advancements in biologic therapies are significantly transforming the chronic urticaria market, offering hope for patients with this often debilitating condition. Chronic urticaria, characterized by persistent hives and itching, has traditionally been challenging to manage, with many patients finding limited relief from conventional treatments. However, the emergence of biologic therapies is revolutionizing this landscape. Biologics, designed to target specific components of the immune system, are showing promising results in controlling chronic urticaria symptoms. Omalizumab, an anti-IgE monoclonal antibody, has been a game-changer, providing substantial symptom relief for many patients. Its success has spurred further research into other biologic agents, such as ligelizumab, which targets IgE with higher affinity, potentially offering even greater efficacy.
Request a PDF Sample Report: https://www.imarcgroup.com/chronic-urticaria-market/requestsample
Moreover, advancements are not limited to anti-IgE therapies. Interleukin (IL) inhibitors, such as dupilumab (an IL-4 and IL-13 inhibitor), are being explored for their role in treating chronic urticaria, given their success in other allergic conditions. These developments reflect a growing understanding of the complex immunopathology underlying chronic urticaria, allowing for more targeted and effective interventions. The trend toward personalized medicine is also gaining momentum in this field. Genetic and biomarker studies are helping to identify which patients are most likely to respond to specific biologic therapies, optimizing treatment outcomes. Additionally, ongoing clinical trials are expanding the repertoire of biologic options, with several promising candidates in various stages of development. Overall, the advancements in biologic therapies are ushering in a new era of treatment for chronic urticaria, offering improved quality of life for patients. Continued research and innovation hold the promise of even more effective and personalized therapeutic options in the near future.
Increased Focus on Patient-Centric Care: Contributing to Market Expansion
The chronic urticaria market has seen a significant shift towards patient-centric care, reflecting a broader trend within the healthcare industry. This approach prioritizes the needs, preferences, and experiences of patients, aiming to enhance their overall well-being and treatment outcomes. Chronic urticaria, characterized by recurrent hives and itching, profoundly impacts patients’ quality of life, necessitating a care model that extends beyond traditional symptom management. Key aspects of this patient-centric focus include personalized treatment plans, improved patient education, and the incorporation of patient feedback into care strategies. Advances in biotechnology and pharmacology have enabled more tailored therapies, addressing individual patient profiles and specific disease patterns. Healthcare providers are increasingly utilizing tools such as mobile health apps and telemedicine to facilitate continuous patient engagement and real-time monitoring, ensuring that treatment plans are dynamically adjusted to meet evolving needs.
Patient education has also become a cornerstone of chronic urticaria management, empowering patients with knowledge about their condition, treatment options, and lifestyle modifications that can help mitigate symptoms. This education fosters a collaborative relationship between patients and healthcare providers, enhancing adherence to treatment regimens and improving outcomes. Moreover, the integration of patient-reported outcomes and experiences into clinical practice and research has become crucial. By actively involving patients in the decision-making process, healthcare providers can better understand the nuanced impacts of chronic urticaria on daily life, leading to more empathetic and effective care. Overall, the increased focus on patient-centric care in the chronic urticaria market signifies a transformative shift towards holistic, personalized, and responsive healthcare, ultimately aiming to improve the quality of life for those affected by this challenging condition.
Technological Innovations and Digital Health:
Technological innovations and digital health are revolutionizing the chronic urticaria market, offering new hope for patients and healthcare providers alike. Chronic urticaria, a condition characterized by persistent hives and itching, has traditionally been challenging to manage due to its unpredictable nature and the need for individualized treatment plans. However, recent advancements in technology are transforming this landscape. Telemedicine and mobile health applications are enabling real-time monitoring and management of symptoms, allowing patients to receive timely advice and interventions without the need for frequent in-person visits. These digital tools also facilitate better patient education and adherence to treatment regimens, improving overall outcomes.
Moreover, artificial intelligence (AI) and machine learning (ML) are being harnessed to develop predictive models that can forecast flare-ups, enabling preemptive treatment and reducing the severity of symptoms. AI algorithms analyze vast amounts of patient data, identifying patterns and triggers that might be missed by conventional methods. This personalized approach ensures that patients receive more targeted and effective treatments, minimizing trial-and-error and enhancing quality of life. Wearable devices and smart sensors are also playing a crucial role in the chronic urticaria market. These gadgets continuously monitor physiological parameters such as skin temperature and hydration levels, providing valuable insights into the patient’s condition and response to treatments. Integration of these devices with digital health platforms ensures seamless data collection and analysis, facilitating proactive and informed clinical decisions. In summary, technological innovations and digital health are significantly impacting the chronic urticaria market by improving diagnosis, management, and treatment outcomes. These advancements not only enhance patient care but also pave the way for more efficient and personalized healthcare solutions, ultimately transforming the lives of those affected by this chronic condition.
Buy Full Report: https://www.imarcgroup.com/checkout?id=6725&method=587
Leading Companies in the Chronic Urticaria Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global chronic urticaria market, several leading companies are at the forefront of research, development, and commercialization of treatments. Some of the major players include Novartis, Merck & Co., and Sanofi. These companies are leveraging advanced technologies and innovative approaches to address the unmet needs of patients with chronic urticaria.
Novartis has announced positive results from its Phase III REMIX-1 and REMIX-2 studies for remibrutinib, a Bruton’s tyrosine kinase (BTK) inhibitor. These studies showed rapid and significant improvements in chronic spontaneous urticaria (CSU) symptoms as early as two weeks after treatment initiation. If approved, remibrutinib could become the first new class of CSU treatment in a decade, offering a new option for patients not adequately controlled by H1-antihistamines.
Moreover, Merck has made significant progress in its drug development efforts. The company has been focusing on novel treatments for chronic conditions, including chronic urticaria. Additionally, Merck has received FDA approval for WINREVAIR (sotatercept-csrk), a first-in-class treatment for pulmonary arterial hypertension (PAH), which underscores Merck’s commitment to innovative therapies for chronic conditions.
(sotatercept-csrk), a first-in-class treatment for pulmonary arterial hypertension (PAH), which underscores Merck’s commitment to innovative therapies for chronic conditions.
Apart from this, Sanofi’s rilzabrutinib, an oral BTK inhibitor, has shown promising results in a Phase 2 trial, significantly reducing itch and disease activity in adults with moderate-to-severe CSU who did not respond adequately to H1 antihistamines. This rapid improvement was observed as early as the first week of treatment. These findings will support the initiation of Phase 3 trials later in 2024.
Request for customization: https://www.imarcgroup.com/request?type=report&id=6725&flag=E
Regional Analysis:
The major markets for chronic urticaria include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for chronic urticaria while also representing the biggest market for its treatment. This can be attributed to the increasing prevalence and awareness, advanced healthcare infrastructure, and ongoing R&D investment.
Moreover, chronic urticaria affects about 0.5-1% of the population globally, and the U.S. accounts for a considerable share of this demographic. The prevalence of chronic urticaria in the U.S. is significant, affecting a substantial number of individuals. Additionally, there is a heightened awareness among healthcare professionals and patients about chronic urticaria. This has led to better diagnosis rates and more patients seeking treatment.
Besides this, the U.S. has a well-developed healthcare system with extensive access to medical care, including specialized treatments for dermatological conditions like chronic urticaria. This infrastructure supports comprehensive diagnosis, treatment, and management of the condition, enhancing the overall therapeutic landscape.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the chronic urticaria market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the chronic urticaria market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current chronic urticaria marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/chronic-urticaria-market
IMARC Group Offer Other Reports:
Orphan Drugs Market: The global orphan drugs market size reached US$ 214.8 Billion in 2023, and expected to reach US$ 541.3 Billion by 2032, exhibiting a growth rate (CAGR) of 10.5% during the forecast period from 2024 to 2032.
Spinal Implants and Surgery Devices Market: The global spinal implants and surgery devices market size reached US$ 11.9 Billion in 2023, and expected to reach US$ 17.4 Billion by 2032, exhibiting a growth rate (CAGR) of 4.2% during the forecast period from 2024 to 2032.
Chromatography Resins Market: The global chromatography resins market size reached US$ 2.4 Billion in 2023, and expected to reach US$ 3.9 Billion by 2032, exhibiting a growth rate (CAGR) of 5.7% during the forecast period from 2024 to 2032.
Sepsis Diagnostics Market: The global sepsis diagnostics market size reached US$ 687.2 Million in 2023, and expected to reach US$ 1,404.3 Million by 2032, exhibiting a growth rate (CAGR) of 8.3% during the forecast period from 2024 to 2032.
Breast Imaging Market: The global breast imaging market size reached US$ 4.5 Billion in 2023, and expected to reach US$ 8.8 Billion by 2032, exhibiting a growth rate (CAGR) of 7.5% during the forecast period from 2024 to 2032.
Surgical Robots Market: The global surgical robots market size reached US$ 5.4 Billion in 2023, and expected to reach US$ 20.7 Billion by 2032, exhibiting a growth rate (CAGR) of 15.7% during the forecast period from 2024 to 2032.
Life Sciences Bpo Market: The global life sciences BPO market size reached US$ 413.0 Billion in 2023, and expected to reach US$ 804.2 Billion by 2032, exhibiting a growth rate (CAGR) of 7.46% during the forecast period from 2024 to 2032.
Per Diem Nurse Staffing Market: The global per diem nurse staffing market size reached US$ 8.7 Billion in 2023, and expected to reach US$ 13.1 Billion by 2032, exhibiting a growth rate (CAGR) of 4.73% during the forecast period from 2024 to 2032.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
Phone Number: – +1 631 791 1145, +91-120-433-0800