The Stelo glucose meter. (Image courtesy of Dexcom)
The Stelo glucose meter. (Image courtesy of Dexcom)
Dexcom (Nasdaq:DXCM) chairman, president and CEO Kevin Sayer says the prospect of an over-the-counter CGM created an atmosphere of unease.
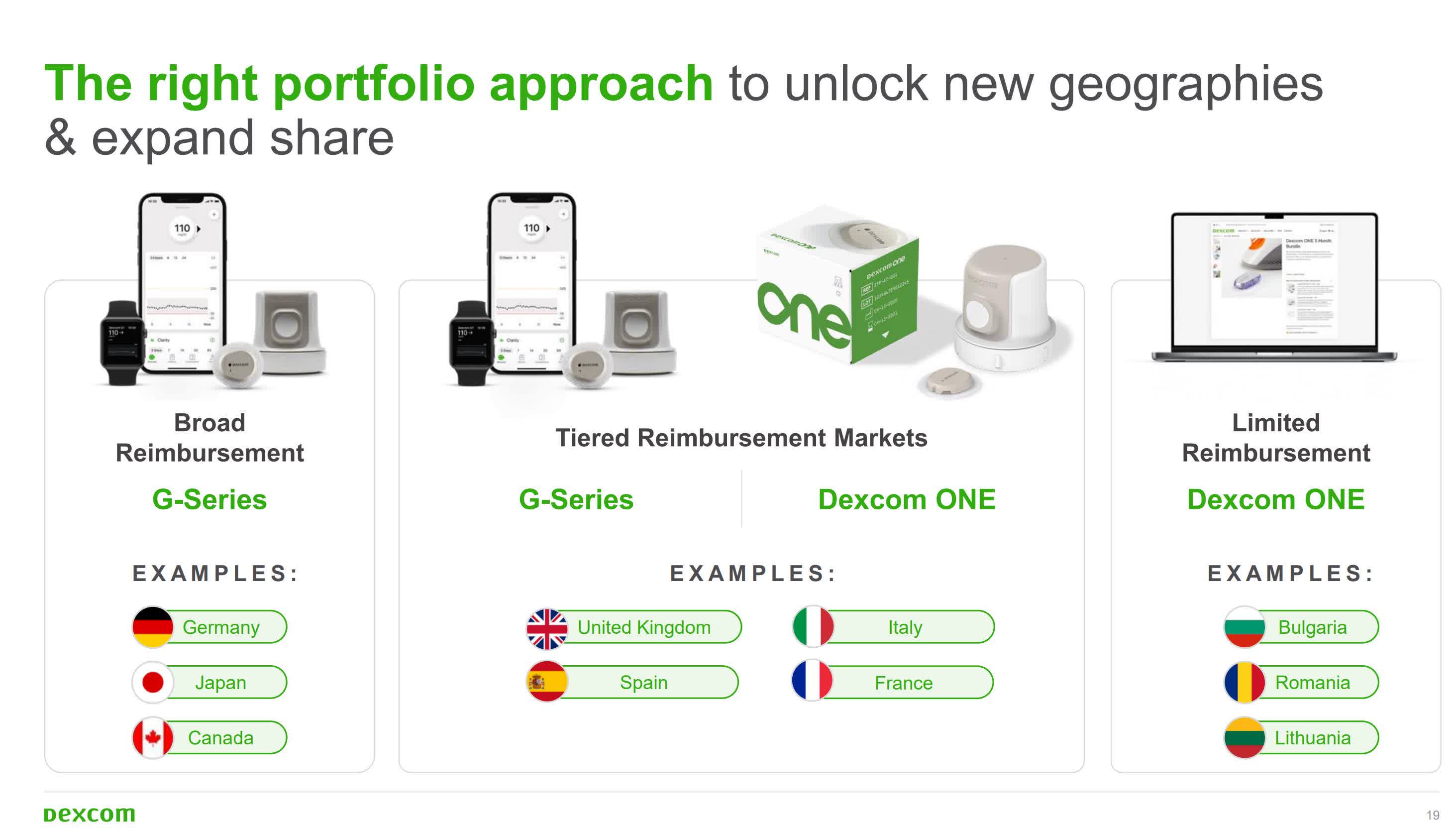
When the company first began planning for a glucose monitor suitable for the type 2 diabetes population not taking insulin, there were questions about reimbursement, the impact of GLP-1 drugs, and more reasons for some form of care.
“It made us all uncomfortable,” Sayer said. “Honestly, it made our whole company uncomfortable. There have been many pro and con debates about this.”
Dexcom announced a new sensor product at an investor day just a year ago. In January, the company submitted the sensor, called Stelo, to the FDA. At the time, however, they remained tight-lipped about any potentially notable clue.
Stelo became the first CGM to receive over-the-counter approval from the FDA less than two months later in March. The approval took only 75 days.
Dexcom estimates that approximately 25 million people in the US with type 2 diabetes who do not use insulin but could benefit from using CGM. Speak with Business news about medicine delivery at the American Diabetes Association Scientific Sessions in Orlando, Florida, Sayer said Stelo is an option the company is eager to bring to market.
“Again, we’re the first here — we got that first approval, and that’s a part of our culture that’s been very important to us,” Sayer said. “We decided it was very important for this group. We are very comfortable now. We certainly made the right choice. Maybe there will be some pain in the next year or two when we find out. I hope not, but I know that in five years we will see this as a milestone in our industry.”
More about Stelo and Dexcom’s plans for the new sensor
The small, wearable sensor is intended for people 18 years and older who are not using insulin therapy. The sensor is worn on the back of the arm and transmits insights directly to the user’s smartphone.
Stelo offers a gestation period of 15 days based on the G7 platform. It offers a software experience tailored specifically for non-insulin users. The company said it makes it easier for the target group to access CGM and provides an option for those who do not have insurance coverage for CGM.
Initially, Dexcom plans to buy Stelo online without a prescription, with a launch scheduled for late August. As for potential competition, Abbott received FDA approval for its over-the-counter Libre Rio earlier this month. Libre Rio focuses on adults with type 2 diabetes who do not use insulin and who manage diabetes through lifestyle changes.
The move to over-the-counter seems to be popular, and for good reason.
 The Stelo glucose meter for people with diabetes who do not use insulin. (Image courtesy of Dexcom)
The Stelo glucose meter for people with diabetes who do not use insulin. (Image courtesy of Dexcom)
“We will build on the experience of going over the counter,” Sayer said. “We wanted to take a group of patients who didn’t want to go to their doctor to get a prescription – we wanted them to be able to just order it. And in the beginning we wanted to control distribution a little bit.”
The initial launch will take place via a Dexcom-controlled website, but the company is doing everything it can to prepare for a wider launch. Sayer said it is building “boatloads” of inventory before rolling out the sensor.
Dexcom wants to target “25 million people with diabetes who need a better tool now,” Sayer explains. Over time, it plans to collect data and demonstrate the improvements in the lives of these people. He also hopes for reimbursement based on that potential data.
According to Sayer, fighting long-term complications is the target group’s biggest goal in living with type 2 diabetes. Someone currently dependent on insulin must worry about controlling glucose levels in the present. A person who doesn’t use insulin may not suffer from complications today, but Sayer says he or she worries about blindness, heart attacks, kidney failure and amputations.
“If they can lower their A1c and increase their time in range, that significantly extends their lives and makes their medications more valuable to them,” Sayer said. “It will be a very good product for us.”
The new direct-to-watch feature highlights a number of G7 improvements
Earlier this month, Dexcom launched a new capability: the latest generation G7 CGM can now connect directly to the Apple Watch in the US.
Upon its official launch, the G7 was the first and only CGM available with a direct-to-Apple-Watch feature. It gives users the freedom and convenience of real-time glucose readings, even without an iPhone at hand.
Sayer said this feature was seven years in the making.
“It’s been incredibly well received by the community,” he says. “We purposely designed our system so that (users) can get their data wherever they want.”
The happiest group, he says, are athletes who no longer need to carry their phones with them to receive glucose data while exercising. Another demographic is mothers sending their diabetic child to school with a cell phone to monitor their glucose. Now they don’t have to do that.
Dexcom is working on more options around mobile connectivity to make the integration full-service.
“Our first group of users at the time were very strict, compliant people with type 1 diabetes,” says Sayer. “As we entertain more and more patient groups, we need to meet them where they are and give them the features that make it easy for them to use. And direct-to-watch is one they’ve been clamoring for for a long time. We are very happy that we can finally release it.”
The G7 innovation doesn’t stop there, however. Dexcom has also added medication and event logging capabilities, while expanding the G7’s connectivity range and making software revisions for quick reconnection if the CGM loses connectivity to its companion device.
“These are pretty big software features,” Sayer said. “We’ve literally updated that app every four to six weeks since launch. It’s a good piece of software and much better than what we had as our software team has really grown and matured.”
Dexcom is also making progress in automated insulin delivery
The past seven months have brought significant developments for Dexcom in the field of automated insulin delivery integration. For years, commercial automated pumps were paired with the G6, but when the G7 hit the market in early 2023, companies had to work to bring the new combination to the diabetes population.
“We are always happy to get G7 to these users,” Sayer said. “They’ve been doing very well with G6 for quite some time, but G7 just offers a much better experience from a wear perspective. It is still the most accurate sensor on the market, and will remain so. The more accurate the data you enter into the system, the better the care you will receive, and G7 is noticeably better than G6, at least clinically.”
First, in December, Tandem Diabetes Care announced the integration of G7 with its t:slim X2 pump. Beta Bionics began rolling out its iLet bionic pancreas with G7 a day later. Tandem later added G7 to its second pump, the small, durable Mobi system, last month. Finally, last week, Insulet began the full launch of its latest generation Omnipod 5 with G7 in the US.
“We know that people who use insulin with CGM do better,” Sayer said. “We know that people with (automated insulin delivery) systems do better. The systems are gradually getting better.”
Sayer said the next step is to see how the algorithms evolve and possibly learn from individual physiology. Over time, he said automated insulin technology could include more of these features to better personalize results.
He believes that with G7 and the latest pumps, users will have better data and the systems will be better controlled and perform at a higher level.
“Overall, their data will be better and these systems will be well managed and do very well,” Sayer said. “The integrations have been very good.”





/medriva/media/post_banners/content/uploads/2024/01/dexcom-stelo-cgm-continuous-glucose-monitor-20240113194540.jpg)
:format(webp)/cdn.vox-cdn.com/uploads/chorus_asset/file/25219925/IMG_3070.jpg)









.jpg)