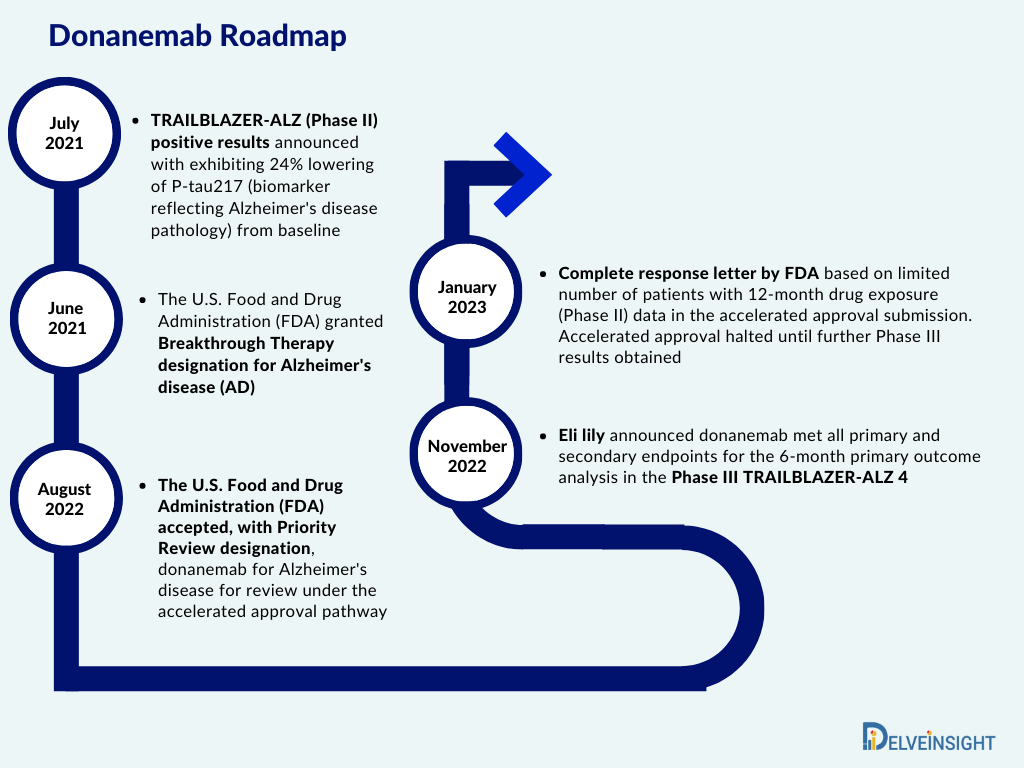
Lilly’s Alzheimer’s Drug Donanemab Backed by FDA Advisors
A Food and Drug Administration (FDA) advisory committee has voted in favor of recommending Lilly’s Alzheimer’s drug donanemab for approval. The vote marks a significant step forward in the development of a potential new treatment for the devastating disease.
About Donanemab
Donanemab is a monoclonal antibody that targets a protein called amyloid-beta, which is believed to play a role in the development and progression of Alzheimer’s disease. The drug is designed to clear amyloid-beta plaques from the brain, potentially slowing or stopping the disease.
Clinical Trial Results
The committee’s recommendation is based on positive results from a Phase II clinical trial involving over 800 patients with early Alzheimer’s disease. The trial found that donanemab reduced amyloid-beta plaques significantly and led to improvements in cognition and function.
FDA Review Process
The FDA will now review the evidence from the clinical trial and the advisory committee’s recommendation before making a final decision on whether to approve donanemab. If approved, it would be the first new Alzheimer’s drug to be approved in over two decades.
Implications for Patients
Donanemab has the potential to provide a much-needed treatment option for patients with early Alzheimer’s disease. By slowing or stopping the progression of the disease, the drug could improve patients’ cognitive function and quality of life.
Controversies and Uncertainties
While the FDA advisory committee’s recommendation is a positive step, some experts have raised concerns about donanemab’s potential side effects, such as brain swelling and bleeding. The long-term safety and efficacy of the drug will need to be evaluated in further studies.
Conclusion
Lilly’s Alzheimer’s drug donanemab has received a favorable recommendation from FDA advisors, opening the door to potential approval. If approved, the drug could revolutionize the treatment of Alzheimer’s disease and provide hope to millions of patients and their families. However, further research is needed to assess the long-term safety and efficacy of donanemab before it can be widely used in clinical practice.This HTML code appears to be related to the online news article about the approval of a drug for early-onset Alzheimer’s disease by Eli Lilly. Let’s break down the elements of the code:This HTML code appears to be related to the online news article about the approval of a drug for early-onset Alzheimer’s disease by Eli Lilly. Let’s break down the elements of the code: 1. `
`: These are paragraph tags used to group related text together. 2. “: This is a “ element with an empty `id` attribute. It’s typically used to create a container for content or to apply styles to a specific section of a web page. 3. `
aFood and Drug Administration advisers voted 11-0 on Monday to approve a drug for early-onset Alzheimer’s disease made by Eli Lilly, ruling that the treatment’s ability to slow cognitive decline in patients was greater than its safety risks.
`: This paragraph contains information about the approval of a drug by Eli Lilly for early-onset Alzheimer’s disease. The opening `` tags with the “big-cap-wrap” and “big-cap” classes are likely used to style the first letter of the paragraph as a large capital letter. 4. `
The daylong advisory panel’s unanimous outcome was the best-case scenario for Lilly, making it likely that the FDA will approve the drug, called donanemab, for a broad population of people diagnosed with mild cognitive impairment due to the disease of Alzheimer’s. A decision is expected later this year.
`: This paragraph provides additional details about the advisory panel’s recommendation and the expected FDA approval for the drug named donanemab. 5. `
“The benefits outweigh the risks, as long as the risks are monitored,” said Kathleen Poston, a neurologist at Stanford University and a member of the advisory panel.
`: This paragraph includes a quote from a neurologist on the advisory panel, highlighting the balance between the drug’s benefits and risks. 6. “: This “ element marks the beginning of a section with restricted content. 7. “: This “ element contains information about the restricted content, indicating that it is an exclusive STAT+ subscriber story. 8. `
STAT++Exclusive story
`: This text further emphasizes that the content is exclusive to STAT+ subscribers. 9. `
Do you already have an account? Log in
`: This text prompts existing subscribers to log in to access the restricted content. 10. `

`: This image serves as a background element for the restricted content section. 11. “: This “ element wraps the remaining restricted content. 12. `
`: This empty `` element is likely related to the paywall system for the restricted content. 13. `
`: This image displays the STAT+ logo within the restricted content wrapper. 14. `
This article is exclusive to STAT++ subscribers
`: This heading indicates that the full article is only accessible to STAT+ subscribers. 15. `
Unlock this article–plus daily news coverage and analysis from the biotech sector–by subscribing to STAT+.
`: This subheading provides additional information about the benefits of subscribing to STAT+ to access the exclusive content. 16. `
Do you already have an account? Log in
`: This text再次提示 existing subscribers to log in to access the restricted content. 17. `
Do you already have an account? Log in
`: This text is similar to the previous one but likely intended for mobile devices. 18. “: This “ element contains information about subscription plans. 19. “: This “ element allows users to toggle between individual and group subscription plans. 20. `Individual plans`: This button represents the individual subscription plan option. 21. `Group plans`: This button represents the group subscription plan option. 22. `
View all subscriptions
`: This text link allows users to view all available subscription options. 23. “: This “ element contains a message encouraging users to subscribe to access unlimited content and exclusive events. 24. “: This “ element wraps the subscription message. 25. “: This “ element contains the subscription message text itself. 26. `
`: This `
` element contains the message encouraging users to subscribe for unlimited access and exclusive benefits. 27. `
Get unlimited access to award-winning journalism and exclusive events.
`: This text highlights the benefits of subscribing to STAT+. 28. `
Subscribe
`: This text is a call-to-action, encouraging users to subscribe. 29. “: This script tag contains JavaScript code used for analytics and tracking purposes. It includes functions for tracking events and conversions related to the subscription and content access.
FDA Advisors Back Lilly’s Alzheimer’s Drug Donanemab
A panel of FDA advisors has recommended approval of Lilly’s Alzheimer’s drug donanemab, offering hope to patients with the debilitating disease. Donanemab targets a protein called amyloid beta, which is believed to accumulate in the brain and contribute to Alzheimer’s development. In clinical trials, the drug reduced amyloid beta levels by 80% and slowed cognitive decline by 30%. “This is a significant finding that could have a major impact on the lives of patients and their families,” said Dr. Howard Fillit, a member of the FDA advisory panel. However, the advisors also raised concerns about the drug’s safety. In clinical trials, some patients experienced side effects such as brain swelling and microbleeds. The FDA is expected to make a final decision on donanemab’s approval by March 7, 2023. If approved, it would be the first new Alzheimer’s drug in nearly 20 years. Analysts estimate that donanemab, if approved, could generate billions in sales for Lilly. The company is already planning to ramp up production to meet potential demand. Alzheimer’s disease is the most common form of dementia, affecting an estimated 6.5 million Americans. Current treatments can only provide temporary relief from symptoms, but donanemab offers the possibility of slowing the progression of the disease. The FDA’s decision on donanemab is eagerly awaited by patients, researchers, and drug companies alike. If approved, it would represent a major breakthrough in the fight against Alzheimer’s disease.













/cloudfront-us-east-2.images.arcpublishing.com/reuters/L2DKHLOPPFIAVMOL7XS3J4DTFY.jpg)








